BIOTHERAPEUTICS
Why Antibiotic free process?
This novel strategy for Biotech/ Biosimilar products without using any antibiotics throughout the process, can serve mankind in the following ways:
- In the current scenario, Biopharma and Biotechnology industries are discouraged by regulatory for use of antibiotics in their process.
- Unused antibiotics, after disposal of the culture media is a potential reason to generate multi drug resistant (MDR) strains in the environment.
- As per regulatory requirement, antibiotic clearance needs to be shown, which increases product cost. Hence, antibiotic free process will lead to a cost effective product.
- In our strategy, chance of loss of plasmid copy number is nil, so yield of expression is high and consistency of expression from batch to batch
Ranibizumab is a recombinant humanized IgG1 kappa isotype monoclonal antibody fragment designed for intraocular use. Ranibizumab binds to and inhibits the biologic activity of human vascular endothelial growth factor A (VEGF-A). It is indicated for the treatment of macular edema after retinal vein occlusion, age-related macular degeneration (wet), and diabetic macular edema.
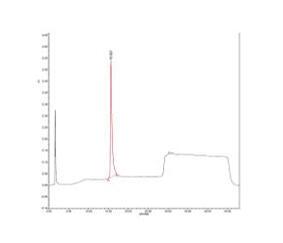
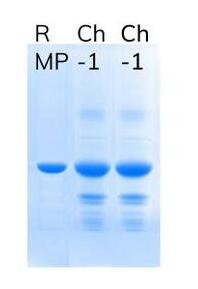
Ameliorate has developed patented process for production of Ranibizumab with ZERO use of Antibiotics starting from clone to final product by using heavy chain and light chain gene cloning for polycistronic RNA.
IP: Reference No: 201741027002
Title: Antibiotic Free process for Production of Biotherapeutics

